Thanks Bill that's very generous.

I'm very pleased you're enjoying the series.
I haven't had a lot of time today but I did manage to get a few slides through dewaxing and into alcohol, ready for staining and mounting. I did get one slide stained & mounted though, a 15µ Carex leaf section the same as in the previous photo's.
This time instead of taking the sections from dewaxing (Histoclear is the wax-solvent I use) to alcohol and staining them with an alcohol-based (in fact 'Cellosolve' as comes ready-mixed) Safranin preparation I used my own (mixed from Safranin powder) aqueous preparation.
This required the additional steps of moving the section from alcohol, via an alcohol/DIW series of decreasing concentrations (of IPA) into water, staining the slide (for 1 hour) with the aqueous (0.5%) Safranin, then moving backwards through the series to return to pure(ish) alcohol, then a series of alcohol/Histoclear mixtures until the slide was in pure Histoclear - the solvent compatible with my intended resinous mounting-medium, 'Numount' - which is a modern replacement for Canada Balsam resin, and doesn't turn yellow over time either...
Once the stained slide is in Histoclear, it is simply taken out of the Histoclear container and put on the bench ready for mounting (just as demonstrated in my video of the same procedure using an alcohol-based mountant). Excess Histoclear is wiped away with a tissue then a couple of drops of mountant (Numount resin) are dropped onto the sections, and a drop is put onto the cover-slip.
The cover-slip is then lowered onto the slide, a few tiny weights are placed onto the cover-slip (I use staples from a normal paper-stapler) and the whole thing left for about an hour before a 'first look' under the 'scope. The slide won't be at its optical best for about another 24 hours, as the solvent needs to dry and the cover-slip to 'tighten' onto the sections, but a quick set of pictures is possible and I've a few here to show you...
These sections show a far superior result to the previously-posted alcohol-based stain - the stain is Safranin in both cases, but the aqueous preparation shown below has stained with far more clarity, contrast and differentiation between cell types than the earlier attempt that has no differentiation and seemed a little 'blunt' in terms of clarity.....
The sections in these pictures are all at 15µ - a little too thick, perhaps 10-12µ would be more certain to achieve the ideal (for my intended application in this case, which is to illustrate cellular organization in the tissue) 1-2 cell thickness....
All are permanently mounted and cover-slipped, but not 100% at their optimum (dry) level yet.
Here's a 2-picture stitch at x20 to give an overview;

- 2-picture stitch
- ws_x20_15micron_carex.jpg (241.64 KiB) Viewed 5773 times
The improved definition and differentiation is immediately apparent I think when compared to the earlier (Wednesday's) pictures.
Here's a (dry) x60 picture that shows a stomate and it's constituent cells on the underside of the leaf (which is uppermost in this picture

);
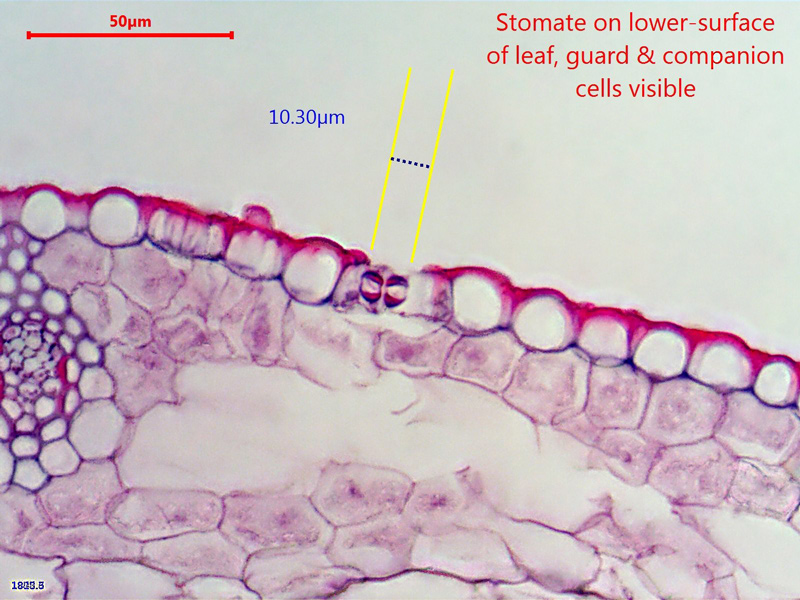
- Not a very crisp picture but a reasonable start
- ws_x60_15micron_carex_(2).jpg (192.21 KiB) Viewed 5773 times
This is a x40 view across the leaf and shows the major difference between the upper (on the left) epidermis and the lower, which also contains stomata, this leaf appears not to have stomata on its upper surface (cannot of course be certain of this from these sections and pictures..) but many regularly-spaced on it's lower surface. the size difference between the upper and lower epidermal cells is also very obvious. Good differentiation throughout - pretty good for a single-stain...
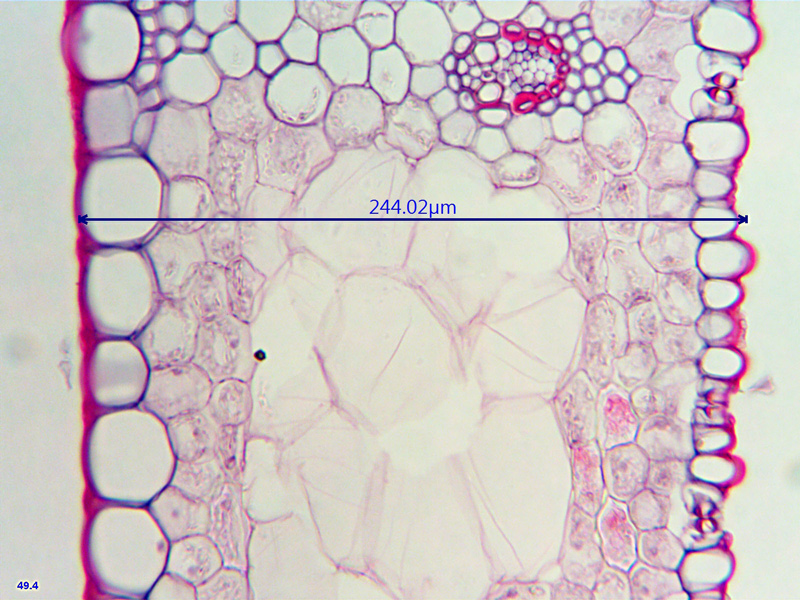
- Across the leaf left = top surface
- ws_x40_15micron_carex_2.jpg (265.84 KiB) Viewed 5773 times
The aqueous Safranin seems to be doing a fine job, plenty of detail & reasonable contrast helps photography.
Next up - a couple of pictures showing some really nice detail of the vascular bundles and attendant sclerenchyma cells that offer strengthening and rigidity perhaps..
This vascular bundle is seen at x40
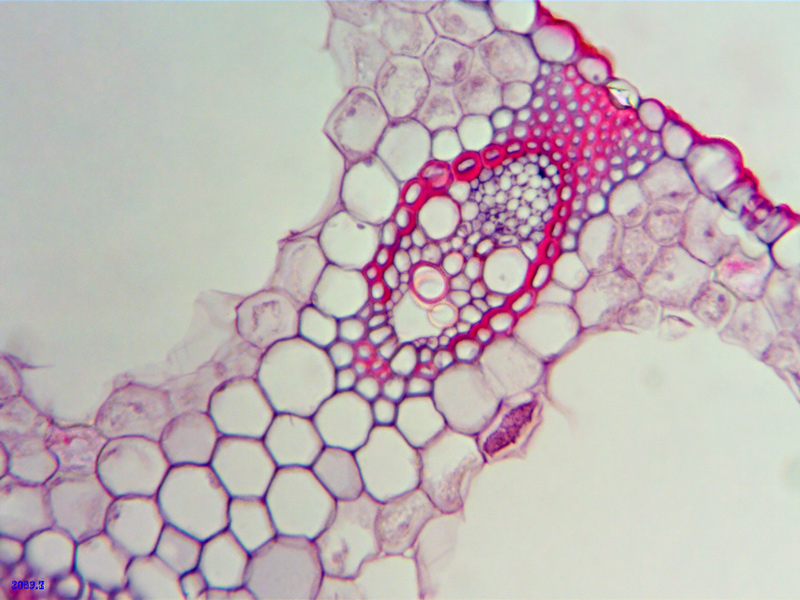
- Vascular bundle midway across leaf
- ws_x40_15micron_carex.jpg (202.35 KiB) Viewed 5773 times
This is a x60 view, not bad clarity of photo' but could be sharperer I think, but a good start.


- Nice detail of bundle's structure
- ws_x60_15micron_carex.jpg (281.87 KiB) Viewed 5773 times
That's about all I've time for tonight, must get some sleep. I'm very pleased with these results, things have improved a lot, tomorrow I hope to get time to stain & mount some more sections and to introduce a 2nd (counter) stain!

What fun, I hope you like them - I certainly love working on them!

The
mighty Shandon has certainly given me a 'leg-up' with my sections - having fun and learning loads!

