As you'll know, I've had recurrent and very troublesome, at times even disappointing, problems achieving good tissue-integrity with any measure of real consistency. Well - my first assumption - using what little knowledge I have and the torn & tattered sections staggering from my microtome's knife, was that the processing was the No.1 suspect - I no longer believe this to be the case for several reasons. The main one being that I've had some sections with very good integrity in the recent past which lead me to tentatively think that at least on occasion, my processing (i.e. chemical) has been good & appropriate.
Then came a happy accidental-discovery - totally unintended and to say the least unanticipated!
Curiosity as usual led me to investigate - a quick look under the stereo 'scope revealed a (partial) section of the historically difficult (or should that be hysterically
Soooo - "Watson - the game's afoot" as they say!
What I did next may sound quite outlandish but here's what I did. I 'stuck' aforementioned razor-blade, in it's unadulterated entirety, onto the hallowed microtome-knife (screaming as I did so
It looks horrific and extremely dubious - here's the peculiar arrangement in all it's glory: Ta-da - sections!
Here are some of the resulting sections as they 'arrived' from my shiny new £0.09 'replaceable microtome knife with integral securing tape'
Upon seeing these rather nice sections I pressed on with a maniacal grin! "To de-waxing we dash". Anyway - I stained & mounted the sections and got the best results for the dreaded Aloe-leaf XS that I have ever achieved - by far. Here's a look: Pretty good for a razor-blade and some sticky tape!
These results and others that have several times duplicated this result with different wax-blocks and specimens have led me to the cautious but not I feel unfounded conclusion that the microtome's knife (specifically it's condition - i.e. damaged by my previous misuse and poor technique) is now my final hurdle or at least very nearly so!
Here are some of those further results which when considered in terms of tissue-integrity would appear to support my current conclusion/s:
The Daffodil flower-stalk (pedicel) that's given me so much trouble, looking pretty good, stained with Fast Green FCF in cellosolve and mounted in alcohol-compatible mountant (as per demo video of earlier post).. Pleased now that I didn't hurl the wax-block into the bin after so many poor sectioning-attempts made with it.
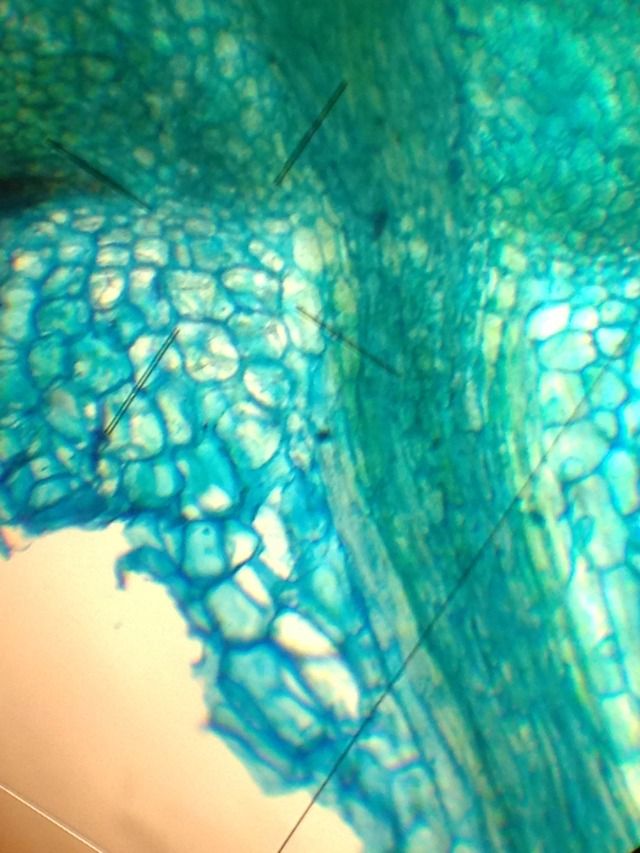
This section exhibits very good integrity and morphology - I wish I'd counter-stained with a nuclear stain to show more intra-cellular detail though - but at this stage I was pretty pleased to have the section at all!
Here's a closer-look - the integrity is good - I'm very pleased and more than a little surprised by my razor-blade results.. In conclusion:
I think I need to buy a new knife for my microtome ASAP.
I think my tissue-processing technique & protocol/s are basically sound and useful.
I will definitely pursue and perfect the replaceable-blade mount using blades bought for about £1.20 for 10! This shouldn't take too long - I'm away for next week but I'll start on it as soon as I get back.
I will start subbing my slides as a matter of course to give better adhesion of the section to the slide's surface, in preparation for the quite harsh de-waxing, staining and mounting stages - no more folding-over for me! I've ordered some Chrome-Alum with which I will make a super-strong subbing-mixture.
Good results at last - albeit by accident and good fortune!
I'll have time for some more sections with this method and perhaps a video of it in action, before the weekend with luck!
Back soon with more details...